What Level of Ammonia Is Toxic to Fish
Introduction
Ammonia causes stress and damages gills and other tissues, even in minor amounts. Fish exposed to low levels of ammonia over fourth dimension are more susceptible to bacterial infections, have poor growth, and will not tolerate routine treatment as well equally they otherwise would. Ammonia is a killer when nowadays in higher concentrations, and many unexplained production losses accept likely been caused past ammonia.
Ammonia accumulates easily in aquatic systems because it is a natural byproduct of fish metabolism. All animals excrete some waste in the process of metabolizing food into the energy, nutrients, and proteins they utilize for survival and growth. In fish, the principal metabolic waste material product is ammonia. Because it is continuously excreted and potentially lethal, successful aquaculture operations must therefore incorporate methods to detect and eliminate ammonia before information technology can accrue and harm fish.
A byproduct of protein metabolism, ammonia is primarily excreted across the gill membranes, with but a small amount excreted in the urine. The decay of uneaten feed and organic thing create small amounts of ammonia, but in most aquaculture systems, fish themselves are the primary source of the compound. The more feed a fish receives, the more than ammonia it will produce. Nevertheless, even a starved fish will produce some ammonia.
Ammonia may exist present in metropolis or well water. Even trace amounts tin can be toxic to fish, and ammonia is colorless, and, in small-scale amounts, odorless. Therefore, the simply way for an aquarist or producer to know if ammonia is present is to examination the h2o.
In water, ammonia occurs in two forms, which together are called total ammonia nitrogen, or TAN. Chemically, these two forms are represented as NH4+ and NH3. NH4+ is called ionized ammonia because it has a positive electric accuse, and NH3 is called united nations-ionized ammonia (UIA) because it has no charge. This difference is important to know because NH3, united nations-ionized ammonia, is the form more than toxic to fish. Both water temperature and pH bear upon which form of ammonia is predominant at whatever given fourth dimension in an aquatic system.
The Nitrogen Cycle
A biological process called the nitrogen bicycle eliminates ammonia from the h2o by converting information technology to other, less toxic compounds (Figure one). The ammonia fish excrete is converted to a chemical compound called nitrite (NO2-) past several genera of leaner, including Nitrosospira and Nitrosomonas. Other groups of bacteria, including Nitrospira and Nitrobacter, convert nitrite to nitrate (NO3-).
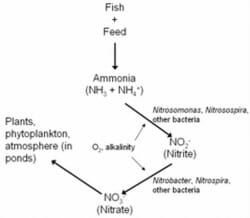
Figure ane. The nitrogen bicycle. Nitrifying leaner use oxygen and alkalinity to catechumen ammonia and nitrite into the less toxic byproduct, nitrate, which is then used by plants or returned to the atmosphere.
In ponds, this process takes place in the surface layers of the mud, and on plants or other structures. In tanks or aquaria, a biological filter, or biofilter, must be provided as a identify where the bacteria can live and flourish. A new biofilter requires 6 to eight weeks to build upward sufficient bacteria to finer reduce ammonia and nitrite levels.
Other of import points to mention about the nitrogen cycle are that both groups of nitrifying bacteria need oxygen and alkalinity to function. If oxygen levels are not sufficient, the process can break down, and ammonia and nitrite levels will increase. Alkalinity (bicarbonate and carbonate) is also used by the nitrifying bacteria. If alkalinity is less than 20 mg/L, the nitrifying bacteria will not be able to part.
It's too of import to annotation that nitrite is toxic to fish at levels as low as 0.x mg/L. If the biofilter is young or impaired, adding chloride in the form of salt (sodium chloride) or calcium chloride at the charge per unit of 10 mg/L chloride for each 1 mg/L nitrite volition reduce the toxic furnishings of nitrite on fish.
Nitrate, the end production of the nitrogen cycle, is considered to be harmless to fish in natural systems and ponds as it is used as a fertilizer past plants, including phytoplankton. In closed systems with piffling or no water exchange, all the same, nitrate will accumulate and may be harmful if college than 250 mg/50.
Ammonia Testing
All aquaculturists and hobbyists should invest in a water quality test kit. A good water quality management programme will reduce fish disease issues, promote growth, and lessen the need for chemical treatments. A h2o quality test kit will pay for itself many times over, both in numbers of fish saved and increased product.
Most commercial ammonia test kits measure the total ammonia nitrogen (TAN). Again, it is the united nations-ionized ammonia (or UIA) portion of the TAN that is more toxic. The UIA fraction of the total TAN can be adamant from the TAN measurement if y'all know the temperature and pH of the water. At high temperatures and loftier pH, there is more than UIA. Therefore, a good ammonia test kit will include a TAN examination, a pH test, and a thermometer.
There are 2 types of ammonia examination kits, and each uses a different testing method to determine TAN. I is the Nessler'southward method and the other is the ammonia salicylate method. If formalin or formalin-containing products have been used within 24-72 hours to treat fish for parasites, the Nessler's method will result in a falsely elevated ammonia reading. Employ of ammonia binding products will besides cause false high ammonia readings with the Nessler'southward method. The reagent used in the Nessler'due south method contains a small corporeality of mercury that in many states must be disposed of as hazardous waste matter.
The other testing method is the ammonia salicylate method. This method is non affected by ammonia binding products or formalin treatments. The ammonia salicylate method is also more authentic than the Nessler's method when testing ammonia in seawater, and information technology does not crave disposal of a chancy waste.
When Should Ammonia Exist Tested?
If stocking densities are high, ammonia should be tested every x to 14 days in ponds, and at least once a week in tanks. If multiple tanks depend upon a common biofilter (i.due east., a recirculating system), at that place is no demand to check every tank individually. Go along records for all tests, and whenever ammonia is found, increase the frequency of testing until the trouble is corrected. Whenever fish are sick, test the water quality.
Ammonia is responsible for more unexplained losses in aquaculture than any other water quality parameter. Equally previously mentioned, information technology is colorless and odorless, so the merely fashion to know if information technology is nowadays is to test for it. Fish submitted to a diagnostic laboratory are tested for diseases (bacteria, parasites, fungi or viruses) merely. Information technology is the responsibility of aquarists and producers to exam the h2o quality, which is very likely to exist the underlying problem.
Interpreting the Ammonia Examination

Effigy 2.

Effigy 3
In healthy ponds and tanks, ammonia levels should always be zero. Presence of ammonia is an indication that the system is out of balance. Therefore, any ammonia in a pond or tank should alert the producer to start corrective measures. Un-ionized ammonia (UIA) is about 100 times more toxic to fish than ionized ammonia.
This UIA toxicity begins as low every bit 0.05 mg/L, and then the result of the TAN test needs to be farther calculated to find the actual concentration of UIA. To practise this calculation, the temperature and pH need to exist measured. One time the pH and temperature are known, the fraction of UIA can exist calculated using a multiplication factor found in Tabular array i. Find the temperature on the top row of the table, and the pH in the left column. The number at which the appropriate cavalcade and row intersect in the table is multiplied by the TAN to give the UIA in mg/L (ppm).
This adding is summarized in Effigy ii and an example is given in Figure three. Anytime the UIA is higher than 0.05 mg/L, the fish are being damaged. As the concentration rises above 0.05 mg/L, it causes more and more damage. At two.0 mg/L, the fish will die. Again, any ammonia indicates a problem in your system. If y'all find it, take corrective measures immediately.
Direction of an Ammonia Problem
The start thing to do when ammonia is present in a swimming or tank is to reduce or eliminate feeding. Fish are not likely to consume during periods of ammonia stress and the uneaten feed will only make the situation worse. Overfeeding is a major cause of loftier ammonia concentrations, and stopping the feeding will allow the natural nitrogen cycle to "catch up" with the nutrient load. If at all possible, a 25 per cent to 50 per cent h2o change will help to remove some of the ammonia. This is but feasible in minor ponds or tanks, so don't try to solve an ammonia problem in a large pond by this method.
Depression levels of dissolved oxygen limit the ability of nitrifying leaner to convert ammonia and nitrite, so it is important to monitor dissolved oxygen.
In ponds, the addition of a phosphate fertilizer may aid to salvage high TAN levels over a flow of days by stimulating phytoplankton growth, which helps remove ammonia from the organization; even so, it may non help quickly enough in an acute ammonia crisis. Utilise a 0–20–0 fertilizer at a rate of 40 pounds per acre. It is of import non to use a fertilizer that contains nitrogen considering nitrogen volition add to the problem. If phosphorus is not a limiting cistron for algal growth in the pond, the phosphate fertilizer method will not work at all.
In tanks without a biofilter, the producer or aquarist should consider incorporating one. Given the vi to eight weeks necessary to establish a biofilter, this volition not help in a crisis, but it is a long-term solution to the trouble.
In the short term, water changes and the use of ammonia bounden products volition alleviate ammonia toxicity. Information technology's important to retrieve that these are short-term solutions. For long-term management, information technology's best to establish a biofilter.
Some chemicals used to treat diseases in fish, especially antibiotics, can be detrimental to the nitrifying bacteria in the biofilter. Both ammonia and nitrite levels should be tested more frequently after applying a disease treatment, to ensure that the biofilter is nonetheless functioning.
Summary
Ammonia is a major waste product of fish and the breakdown of feed and other organics. It tin accumulate in aquaculture or aquarium systems, where it will, at the very least, decrease production. It is often a stressor that leads to illness, and in other cases information technology kills fish directly. The just way to detect its presence is to test for information technology. A fish farmer or aquarist should invest in a water quality test kit, learn how it works, and employ it regularly.
Ammonia examination kits only measure the total ammonia nitrogen (TAN). When this exam indicates a reading in a higher place cypher, producers or aquarists tin can determine the fraction of toxic un-ionized ammonia (UIA) after measuring pH and temperature. The multiplication factors are found in Table i, and an example calculation is found in Figure 3.
When ammonia is nowadays, the fish in the organisation should non be fed until the problem is corrected. In pocket-sized systems, a water change will help, and in large ponds, a 0–20–0 fertilizer may help.
Test for ammonia regularly and take corrective measures equally soon as yous detect it. Severe problems may occur when tests are not performed frequently enough. In one case fish have started to die, it is difficult to correct an ammonia problem without losing more fish.
Ammonia in Aquatic Systems
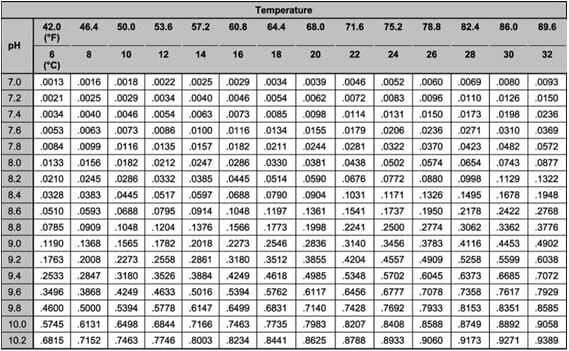
Table one. Fraction of un-ionized ammonia in aqueous solution at different pH values and temperatures. Calculated from data in Emmerson et al. (1975). To summate the amount of un-ionized ammonia present, the Full Ammonia Nitrogen (TAN) must exist multiplied by the advisable gene selected from this table using the pH and temperature from your water sample. Come across the example in Figure 3.
Baronial 2009
Source: https://thefishsite.com/articles/ammonia-in-aquatic-systems
0 Response to "What Level of Ammonia Is Toxic to Fish"
Post a Comment